Catalog of Regulatory Science Tools to Help Assess New Medical Devices
4.6 (521) · € 24.00 · Auf Lager


Regulatory considerations for medical imaging AI/ML devices in the United States: concepts and challenges
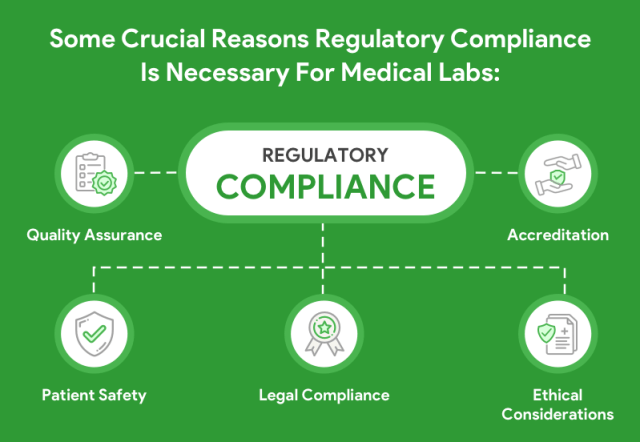
Lab Regulations & Standards for Medical Devices: Enhance Quality

Jiefu Chen - Associate Professor - UH ECE Department

Medical Algorithms Need Better Regulation

Regulatory, Scientific & Quality Conference
동국대학교 바이오헬스의료기기규제과학과

CDRH Regulatory Science Priorities

Medical Device Trials at SCOPE Summit

Medical Device & IVD Regulatory

Regulatory, Scientific & Quality Conference

Regulatory Review of Novel Therapeutics — Comparison of Three Regulatory Agencies

Preclinical CRO Services for Safety Assessment
.png)
FDA Adds New Tools for Evaluating Medical Devices

BHP Centre for Regulatory Science and Innovation – Birmingham Health Partners

Medical devices